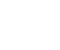Keywords: Quasi Two-dimensional Ferromagnetism Antiferromagnetic Phase Diagram Spin-spin Correlation
Note: This article uses the Sine Scientific Instruments OE1022 lock-in amplifier to make measurements
[Overview]
In 2022, Professor Xiao Dan's team at Sichuan University published an article titled "A Study on Double Inputs Direct Contact and Single Output Capacitively Coupled Conductivity Detector" in Sensors, reporting an improved Double Inputs Direct Contact Single Output Capacitive Coupled Conductivity Detector (DISODCD). This sensor employs dual inputs from contact electrodes, capacitive coupling output from non-contact electrodes, and lock-in amplifier measurements to reduce interference noise signals and amplify gain.
The improved DISODCD utilizes dual inputs from contact electrodes and capacitive coupling output from non-contact electrodes, along with a lock-in amplifier to mitigate interference noise signals and enhance gain. The parallel circuit counteracts some of the adverse capacitive reactance introduced by electrode polarization, reducing the impact of coupling wall capacitance on measuring solution resistance. This sensor lowers the limit of detection (LOD) for analytes and improves the device's sensitivity. The LOD for potassium chloride solution is 1 nM, with an actual detection range for a single sample from 0.01 µM to 10 mM. The response of low-concentration potassium chloride solution to background ultrapure water outperforms that of the Dual Inputs Capacitive Coupling Non-Contact Conductivity Detector (DIC4D) and the Direct Contact Conductivity Detector (DCD) under the same conditions. When the testing cell is contaminated with impurities, the impact of these impurities on DISODCD's response is minimal. In practical applications, the device demonstrates a good lifespan.
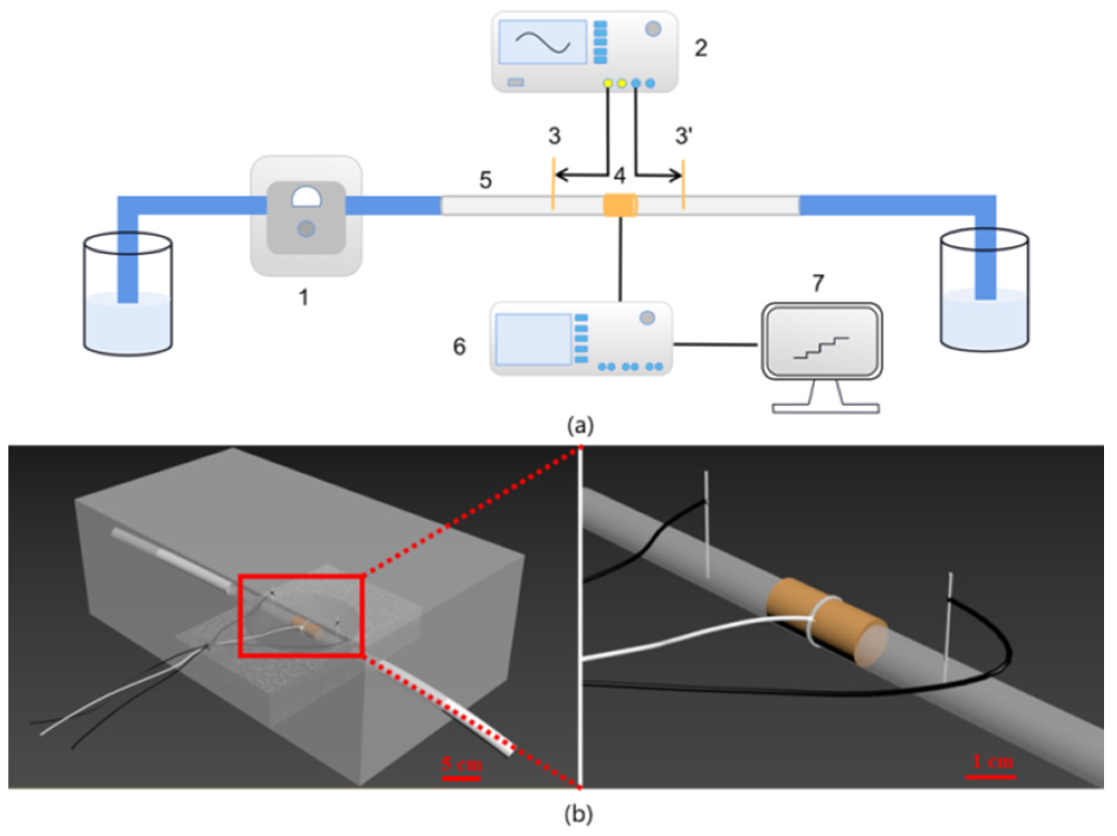
Fig.1 Measurement principle of DISODCD:
(a) Experimental setup design:
(1) Peristaltic pump; (2) Function generator; (3) Input electrode; (4) Output electrode; (5) Plastic tubing; (6) lock-in amplifier; (7) Signal collector.
(b) Model diagram of the inductive section.
[Sample & Test]
The sample solution was prepared using potassium chloride purchased from Shanghai Titan. Ultrapure water (18.2 MW·cm) was sourced from a Millipore water purification system. The testing cell setup consisted of a straw with a diameter of 0.6 cm and a length of 18 cm as the channel, two perforated silver wires with a diameter of 0.3 mm as input electrodes, copper foil as the output electrode, and silicone rubber sealing the pores. The testing cell was fixed within a shielded box. Signal input was provided by a waveform generator (DG4102, Rigol), delivering a sinusoidal signal of up to 20 VPP. The DC output was measured using an AD637 module (Shenzhen Yanglin) and a chromatography data system (N2000, Hangzhou Yijie). An oscilloscope (DS1102E, Rigol) monitored signal strength and waveform variations during the process. To reduce background signal interference and enhance gain, the AD637 module was replaced with a lock-in amplifier (OE1022, Sine Scientific Instruments). A peristaltic pump (Qili constant flow pump) was used to inject samples at an appropriate flow rate. As shown in Figure 1a, the detector's sensor device operates by connecting the signal unit, detection cell, amplifier, and display to measure the concentration of potassium chloride ions. Figure 1b illustrates the schematic of the sensing part of the DISODCD. Ultrapure water was used to prepare potassium chloride solutions of various concentration gradients. During setup, argon gas was introduced into the beaker and volumetric flask to expel internal air, preventing CO2 from dissolving in ultrapure water and forming carbonate ions, which would increase the solution's conductivity and deviate from actual values. Samples should be tested in a relatively sealed environment, and injection should be quick to avoid CO2 dissolution from the air.
In the experiment, a maximum peak voltage of 20 VPP was used, with initial parameters set to 0° in phase. The controlled variable method was employed to select suitable measurement conditions by varying frequency, voltage, phase, and other parameters. The signal-to-noise ratio and low concentration resolution were combined to obtain optimal testing conditions. The specific optimization results for each parameter are shown in the figures below.
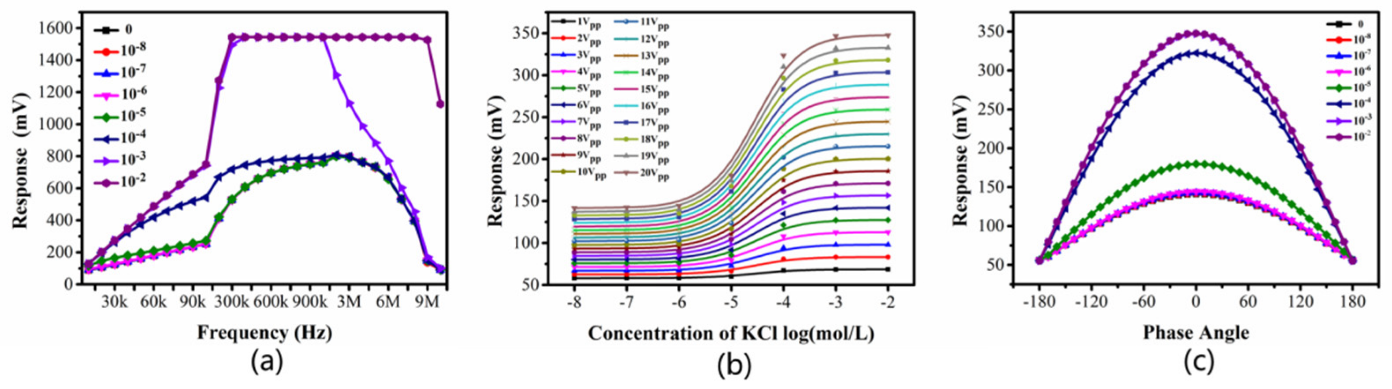
Fig.2 Experimental results of optimising each parameter. (a) frequency; (b) KCI concentration; (c) phase angle.
Fig.3 Signal waveforms of 0-10 mM KCl solution at the input and output of the detection cell. (a) input and output; (b) output.
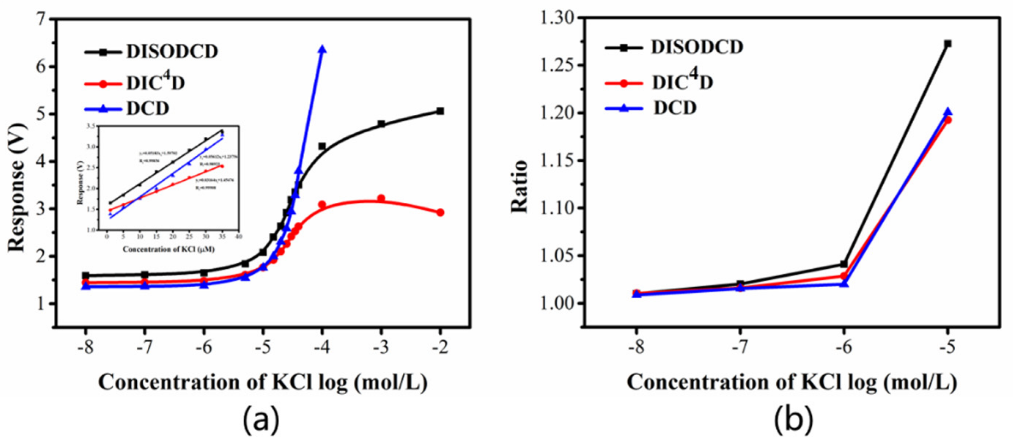
Fig.4 Response of DISODCD, DIC4D and DCD to 0.01 μM-10 mM KCl solution. (a) Response curves of DISODCD, DIC4D, and DCD. (b) Ratio of the responses of DISODCD, DIC4D and DCD.
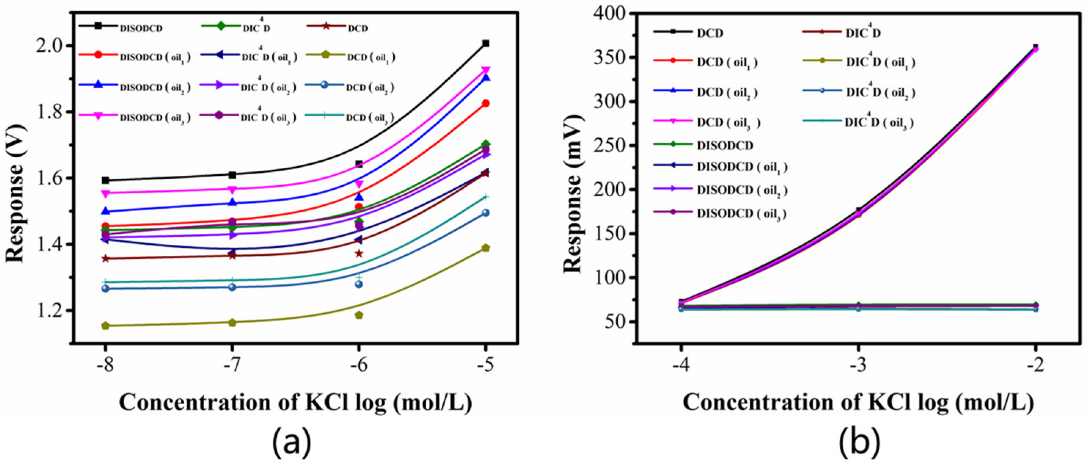
Fig.5 Signal response plots of DCD, DIC4D and DISODCD sensors to KCl solutions interfered with by oil impurities; (a) Response plots of 0.01 ~ 10μM KCl solutions tested 3 times; (b) Response plots of 0.1-10 mM KCl solutions tested 3 times.
[Summary]
In this work, a novel conductivity detection sensor, DISODCD, was constructed. The parallel circuit not only improved sensitivity and resolution but also mitigated some adverse capacitive reactance introduced by electrode polarization, reducing the impact of coupling wall capacitance on the impedance of the measured solution resistance. The actual testing range was from 0.01μM to 10 mM, with a limit of detection (LOD) of 1 nM. The sensor exhibited a response ratio of 1.27 for a 10μM KCl solution compared to ultrapure water, outperforming both the DCD and DIC4D. Experimental results also indicated that impurity contamination had minimal impact on the sensor's performance. This work provides a new approach to enhancing the conductivity measurement performance of sensors and offers a viable solution for measuring the conductivity of organic and inorganic ions.
[Literature]
[Recommended Products]