The molecules of matter are made up of atoms whose chemical bonds are constantly oscillating. When light having a certain number of vibrations is irradiated onto these molecules, the molecules have the property of absorbing such light and rendering it impermeable if the number of vibrations of light is the same as the number of vibrations of some of the molecules therein. If you change the wavelength of the light to scan it, some of the light will be absorbed and you will be able to get the material-specific spectrum. This spectrum is called light absorption spectrum. Like human fingerprints, different substances have different absorption spectra. By measuring the absorption spectrum of an unknown substance, the composition and molecular structure of the substance can be determined.
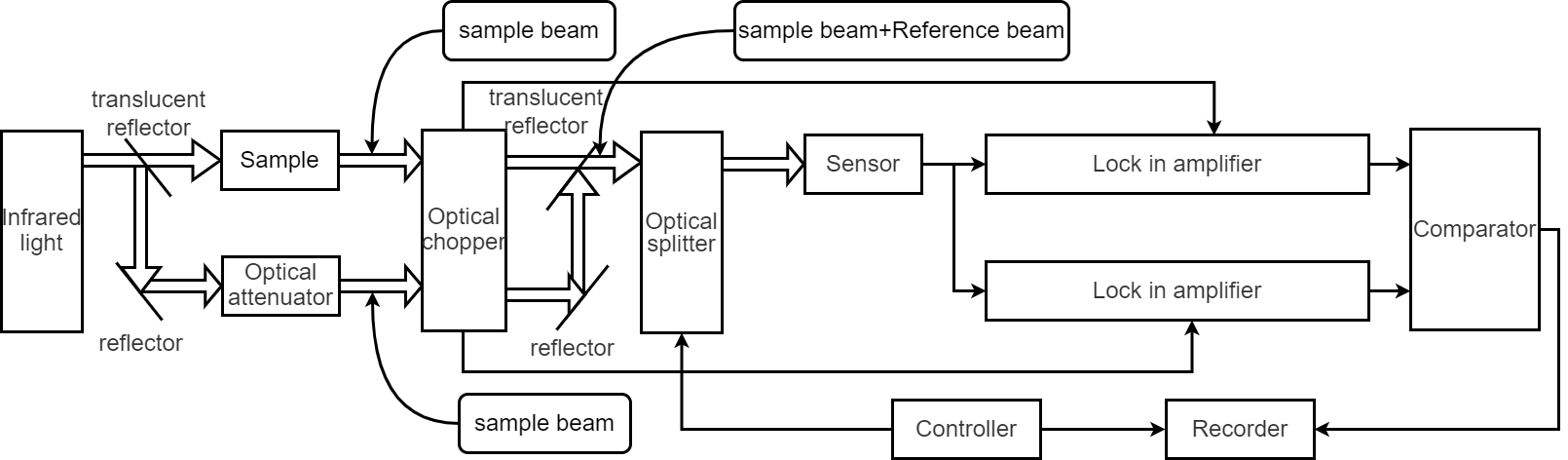
Infrared spectrophotometer is based on this principle to measure. The use of infrared wavelength of 2.5-25um, solid, liquid, gas and other substances in any state can be analyzed.